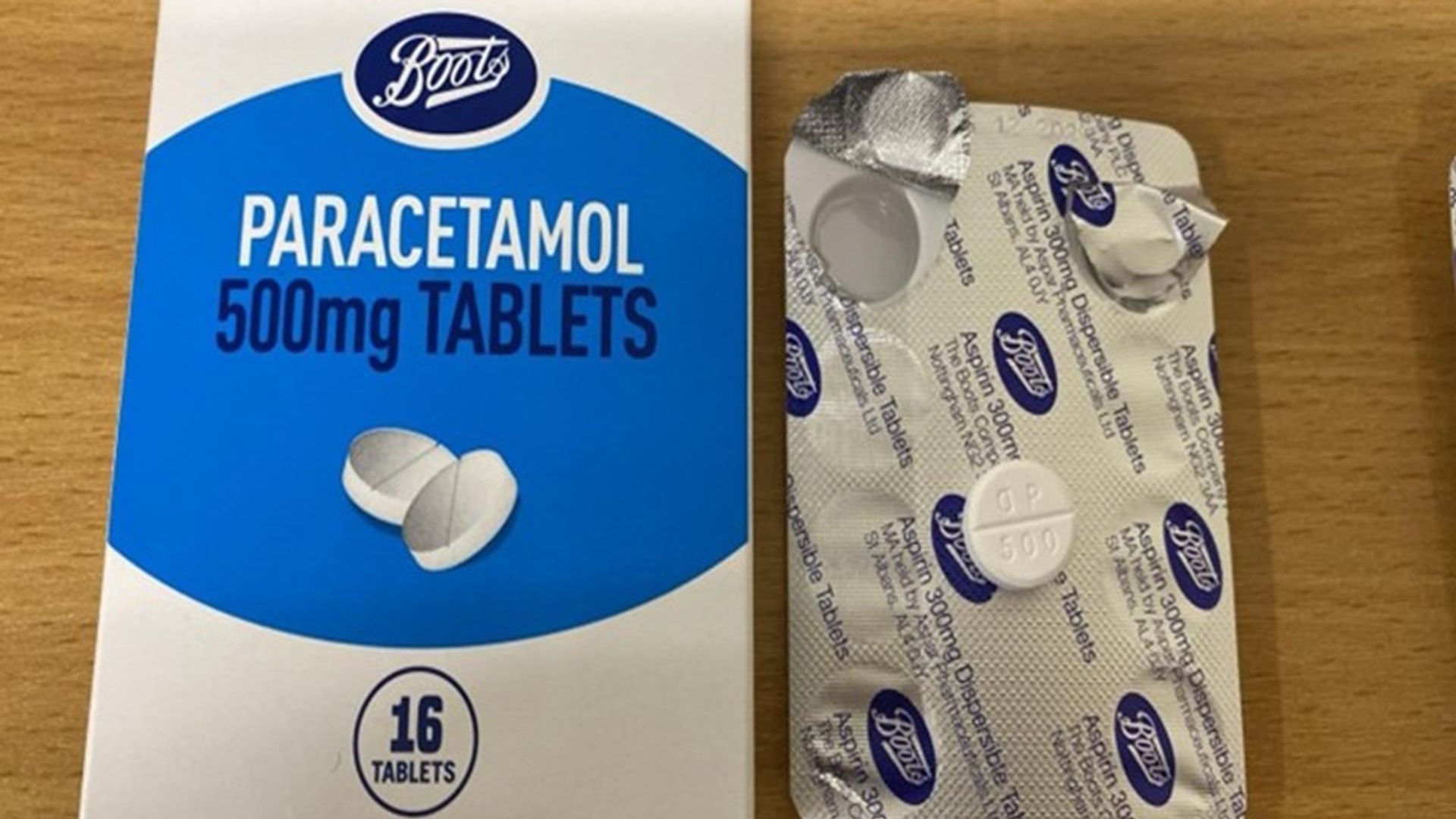
Folks have been instructed to “instantly” cease utilizing packets of paracetamol from Boots after a packaging error.
The affected foil blister packs of 16 tablets inside paracetamol packing containers had been incorrectly packaged as “Aspirin 300mg Dispersible Tablets” regardless of really being 500mg paracetamol tablets.
The UK Medicines and Healthcare merchandise Regulatory Company (MHRA) urged prospects to test if their packs have the batch quantity 241005 on the underside of the field.
“If affected, they need to cease utilizing the product instantly and return it to a Boots retailer for a full refund, with or with out receipt,” the company stated.
The MHRA stated prospects mustn’t preserve the field, even when the error is thought, because it might result in an incorrect dose being taken.
Clients must also alert others if the tablets had been introduced for another person.
Boots additionally posted on its official Fb web page to warn prospects of the recall.
It added the provider, Aspar Prescribed drugs Restricted, confirmed the tablets within the blister packs are paracetamol and never aspirin, and are conducting a “full investigation” into the problem.
Learn extra from Sky Information:
Matt Lucas apologises to Millie Bobby Brown
‘Corrupt’ ex-prison officer jailed
Dr Stephanie Millican, MHRA deputy director profit danger analysis, stated it’s “vitally vital” to test the packaging.
“If the batch quantity is 241005, it’s best to cease utilizing the product and return it to a Boots retailer for a full refund,” she stated.
“In case you are uncertain which pack you’ve bought or have taken Boots Paracetamol 500mg Tablets and skilled any negative effects, search recommendation from a healthcare skilled.”














