
Artificial diamond analysis examines natural molecular interactions underneath the microscope.
Scientists have lengthy developed totally different methods to provide synthetic diamonds, however a brand new technique from researchers, together with a group on the University of Tokyo, provides shocking benefits. By making ready samples in a selected method earlier than exposing them to an electron beam, the group found that their course of not solely helps diamond formation but in addition shields natural supplies from the harm that such beams usually trigger. This breakthrough might open the door to extra superior imaging and evaluation strategies.
Historically, diamond creation depends on remodeling carbon sources underneath excessive bodily circumstances. These embrace pressures of tens of gigapascals and temperatures reaching 1000’s of kelvin, the place diamond stays thermodynamically steady. One other method includes chemical vapor deposition, a technique the place diamond is definitely unstable. In distinction, Professor Eiichi Nakamura and his colleagues on the College of Tokyo’s Division of Chemistry pursued a low-pressure technique that makes use of rigorously managed electron irradiation utilized to a carbon cage molecule often called adamantane (C10H16).
What makes adamantane significantly promising is its structural similarity to diamond. Each share a tetrahedral, symmetrical carbon framework with atoms organized in the identical spatial configuration. This makes adamantane an interesting beginning materials for nanodiamond manufacturing. Nevertheless, profitable conversion is dependent upon the exact removing of adamantane’s C–H bonds to permit new C–C bonds to type, whereas the person constructing blocks assemble right into a three-dimensional diamond lattice. Though this requirement was already identified within the area,
“The actual downside was that nobody thought it possible,” stated Nakamura.
From Principle to Remark
Beforehand, mass spectrometry, an analytical method that types ions in response to their differing mass and cost, had proven that single-electron ionization could possibly be used to facilitate such C–H bond cleavage. Mass spectrometry, nevertheless, can solely infer construction formation within the fuel section, and is unable to isolate merchandise from intermolecular reactions.
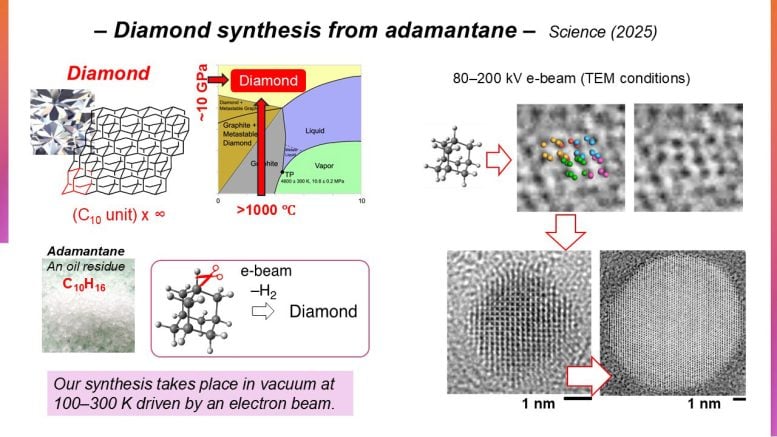
The group was prompted to watch electron-impact ionization of strong adamantane at atomic decision utilizing an analytical and imaging method known as transmission electron microscopy (TEM), irradiating submicrocrystals at 80-200 kiloelectron volts at 100-296 kelvins in vacuum for tens of seconds. Not solely would the tactic reveal the evolution of the polymerized nanodiamond formation, however present highly effective ramifications for the potential of TEM as a device to resolve the managed reactions of different natural molecules.
For Nakamura, who had labored on artificial chemistry for 30 years and computational quantum chemical calculations for 15 years, the research supplied a breakthrough alternative. “Computational knowledge offers you ‘digital’ response paths, however I wished to see it with my eyes,” he stated. “Nevertheless, the widespread knowledge amongst TEM specialists was that natural molecules decompose rapidly as you shine an electron beam on them. My analysis since 2004 has been a continuing battle to point out in any other case.”
The Delivery of Nanodiamonds
The method yielded defect-free nanodiamonds of cubic crystal construction, accompanied by hydrogen fuel eruptions, as much as 10 nanometers in diameter underneath extended irradiation. The time-resolved TEM pictures illustrated the passage of fashioned adamantane oligomers remodeling into spherical nanodiamonds, moderated by the C–H cleavage price. The group additionally examined different hydrocarbons, which didn’t type nanodiamonds, highlighting the suitability of adamantane as a precursor.
The findings open a brand new paradigm for understanding and controlling chemistry within the fields of electron lithography, floor engineering, and electron microscopy. Evaluation of the nanodiamond conversion helps long-standing concepts that diamond formation in extraterrestrial meteorites and uranium-bearing carbonaceous sedimentary rocks could also be pushed by high-energy particle irradiation. Nakamura additionally pointed to the idea it gives for synthesizing doped quantum dots, important to the development of quantum computer systems and sensors.
As the newest chapter in a 20-year-long analysis dream, Nakamura stated, “This instance of diamond synthesis is the final word demonstration that electrons don’t destroy natural molecules however allow them to endure well-defined chemical reactions, if we set up appropriate properties in molecules to be irradiated.” By without end altering the sport in fields using electron beams for analysis, his dream might now present the imaginative and prescient for scientists to uncloud interactions underneath electron irradiation.
Reference: “Fast, low-temperature nanodiamond formation by electron-beam activation of adamantane C–H bonds” by Jiarui Fu, Takayuki Nakamuro and Eiichi Nakamura, 4 September 2025, Science.
DOI: 10.1126/science.adw2025
Funding: JSPS KAKENHI, JST PRESTO
By no means miss a breakthrough: Join the SciTechDaily newsletter.














