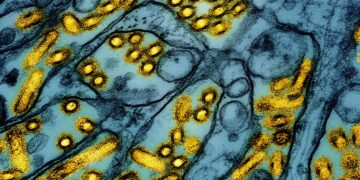
Mind most cancers sufferers will be unable to make use of a brand new drug that slows or stops tumour progress after it was rejected for NHS use.
The Nationwide Institute for Well being and Care Excellence (NICE) has turned down vorasidenib due to uncertainty within the financial information and a scarcity of readability over whether or not the drug improves total survival probabilities, based on draft steering it has revealed.
Vorasidenib is for folks aged 12 or over who’ve had surgical procedure for varieties of low-grade glioma with both the IDH1 or IDH2 genetic mutations.
Present therapy consists of surgical procedure, adopted by radiotherapy or chemotherapy, and sufferers who took vorasidenib in medical trials reported constructive outcomes, together with their tumours shrinking.
The drug, a every day capsule, additionally delayed the time earlier than they wanted one other intervention.
Taylor Pepper, 35, from Peterborough, was recognized with an oligodendroglioma mind tumour in 2024 following a routine eye take a look at, which detected swelling behind her proper eye.
Mrs Pepper, who’s married and has a six-year-old daughter, needed to cease working her enterprise and quit her driving licence.
Surgeons at Addenbrooke’s Hospital in Cambridge eliminated most of her tumour, however couldn’t take all of it due to its location.
Mrs Pepper joined a medical trial of vorasidenib. The drug shrank her tumour, and she’s going to keep on it.
“As a lot as I hold constructive, it is rather scary,” she stated. “However with the therapy, I do know I am in an excellent place.
“With the ability to take this drug has given me much more advantages. I will stay a traditional life and make recollections with my little lady.
“It is devastating that it is a no (from NICE), as a result of mind tumour sufferers have needed to wait a very long time for selections on therapy anyway, so having to attend longer for a call on this may trigger a variety of stress and fear.
“They’ve managed to discover a drug that is not as harsh a therapy as chemo and radiotherapy, which means we’re ready to take action far more.
“Having a mind tumour is tough sufficient, particularly while you’re advised it is incurable and that chemo and radiotherapy would solely make it worse. You’re feeling determined.
“My final MRI scan confirmed a small lower in measurement, which is unbelievable, so I need to keep on this drug because it’s allowed me to hold on as usually as I can.
“Anybody going through this prognosis must be eligible for this drug.”
How frequent are IDH-mutant low-grade gliomas?
IDH-mutant low-grade gliomas are one of the vital frequent major mind tumours recognized in folks aged beneath 50, and about 300 folks in England would have been eligible for vorasidenib had the drug been permitted.
A medical trial on 331 sufferers from 10 nations discovered that vorasidenib slowed and even stopped tumour progress in sufferers with grade 2 IDH-mutant glioma.
Daybreak Emerton, a trustee of Astro Mind Tumour Fund, whose son Shay acquired the drug, stated: “I’m dismayed that NICE has not made it out there on the NHS.
“If NICE reverses this choice, eligible sufferers may expertise improved high quality of life, fewer seizures and delayed therapy with harsher therapies.
“It could additionally show the UK’s dedication to innovation and supply all sufferers with hope for progress in mind tumour therapy.”
Learn extra from Sky Information:
Pair found dead in camper van after cider festival
Social media star to be deported from Australia
Dr Simon Newman, chief scientific officer at The Mind Tumour Charity, stated: “This draft choice could be very disappointing because it means extra sufferers can have chemotherapy and radiotherapy sooner than crucial, which may be efficient, however which might have important long-term unintended effects.
“We’re urgently asking our neighborhood to assist flip this draft ‘no’ from NICE right into a ‘sure’.
“Outcomes for mind tumour sufferers stay among the many poorest of any most cancers, so we have to work with all stakeholders to make sure nice science is translated into medicines which are out there on the NHS as shortly as attainable.”